
Unrecognised Muscle-Bone Crosstalk Helps Prevent Muscle Atrophy and Bone Loss in Astronauts
As the Chinese space program evolves, significant challenges remain, including keeping astronauts healthy by preventing muscle atrophy and bone loss in outer space during long-term space missions. Skeletal muscles (SMs) and bones are intimately involved organs and functionally coupled. Mechanical loading such as exercise increases the muscle mass and bone mineral density simultaneously, while mechanical unloading such as prolonged bed rest or spaceflight results in muscle atrophy accompanied by bone loss. The coupling of SMs and bones are primarily considered as a mechanical interaction-dependent process, in which bone provides an attachment site for SMs and SMs apply load to bone. Recently, researchers have recognized that SMs exert as an endocrine organ capable of secreting a variety of myokines, including humoral cytokines and growth factors for mediating the SM-bone crosstalk to regulate bone metabolism. Apart from myokines, emerging evidence indicates that SMs can also release exosomal myomiRs for controlling muscle development. However, the extent to which the SM-derived exosomal myomiRs contribute to SM-bone crosstalk remains undetermined.
Our preliminary study has documented that the high levels of circulating exosomal myomiRs, including miR-206 and -133b, were associated with the low bone formation rate in HLS mice and the low level of circulating bone formation marker in bedridden patients. The expression profiles of serum exosomal myomiRs from mice with SM-specific knockout of the miRNA-processing enzyme Dicer (SMDicerKO), soleus-specific knockout of Dicer (SolDicerKO) and miR-206-133b double knockout (miR206-133bDKO) further demonstrated that SM-derived exosomal myomiRs dominantly contribute to the elevated circulating exosomal myomiRs in HLS mice. Moreover, we evidenced that the SM-derived exosomal myomiRs could transfer to osteoblasts both in vitro and in vivo. Therefore, we hypothesize that the SM-derived exosomal myomiRs could inhibit osteoblastic bone formation during mechanical unloading-induced muscle atrophy.
In this project, we will investigate whether conditional depletion of myomiRs or specific blockage of exosome secretion in skeletal muscles could attenuate the reduced OBF in mice after mechanical unloading. We will also examine the effect of replenishing the SM-derived serum exosomes with or without myomiRs on OBF in mice after mechanical unloading. This project is expected to establish a previously unrecognized role of SM-derived exosomal myomiRs in muscle-bone crosstalk. More importantly, it would provide novel therapeutic strategies for promoting bone formation during mechanical unloading-induced muscle atrophy, which could be beneficial to the bone health of astronauts in future.
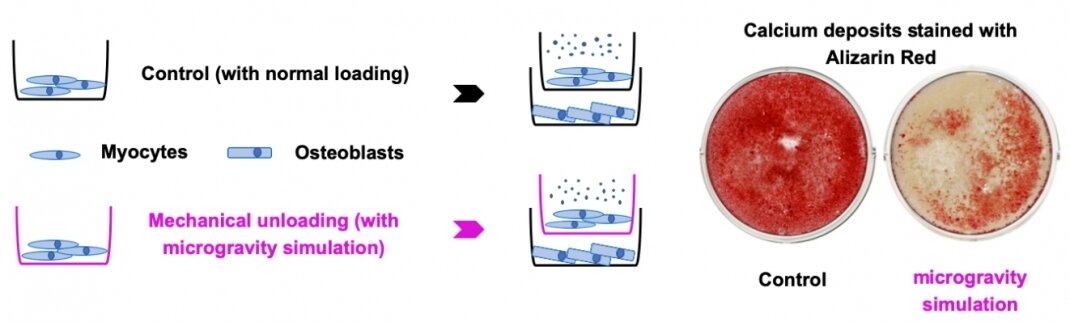
Figure 1.
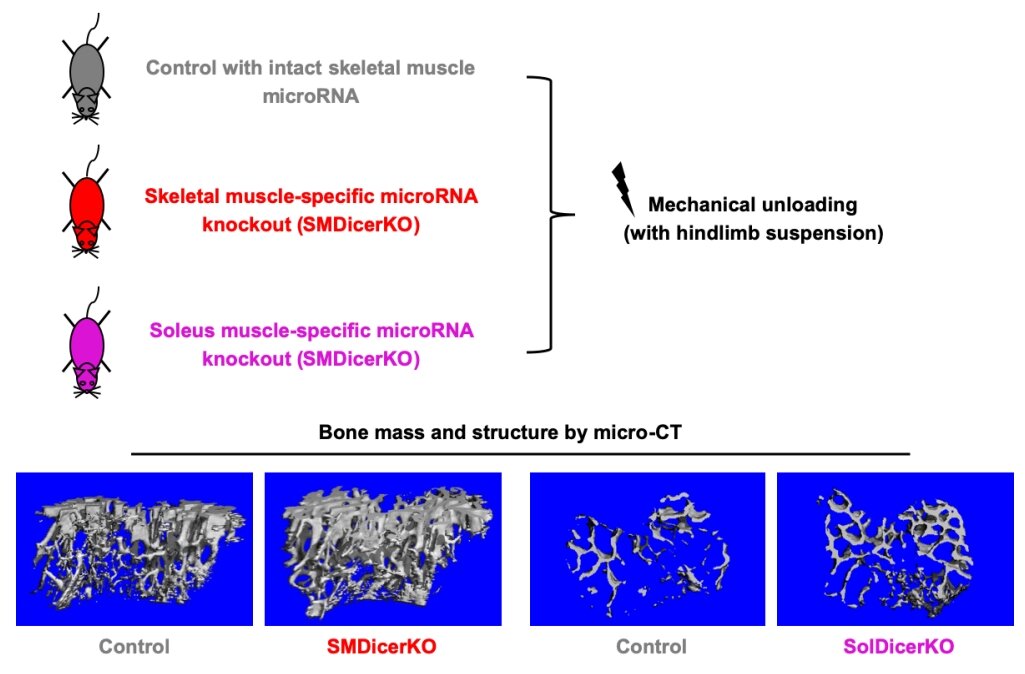
Figure 2.
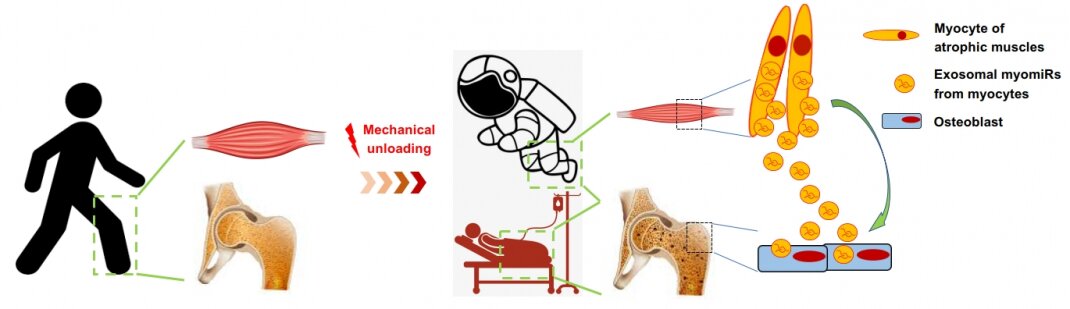
Figure 3.
Research Output
Published articles
- Liu, J., Dang, L., Wu, X., Li, D., Ren, Q., Lu, A., & Zhang, G. (2019). MicroRNA‐mediated regulation of bone remodeling: A brief review. JBMR plus, 3(9), e10213. https://doi.org/10.1002/jbm4.10213
- Zhuo, Z., Wan, Y., Guan, D., Ni, S., Wang, L., Zhang, Z., ... & Zhang, G. (2020). A Loop‐Based and AGO‐Incorporated Virtual Screening Model Targeting AGO‐Mediated miRNA–mRNA Interactions for Drug Discovery to Rescue Bone Phenotype in Genetically Modified Mice. Advanced Science, 1903451. https://doi.org/10.1002/advs.201903451
Awards
- Liu, J., Young Investigator Award, Unraveling the mystery behind bone-cartilage crosstalk: Osteoclast-derived exosomal microRNAs deprive the resistance of cartilage to matrix degeneration, angiogenesis and innervation in osteoarthritis. American Society of Bone and Mineral Research (ASBMR) 2019 Annual Meeting, Orlando, U.S.A.













