
Dr. Angelo H. ALL

Contact Information
Tel: 3411 5956
Email: angelo@hkbu.edu.hk
Website: https://chem.hkbu.edu.hk/Angelo-Lab
Associate Professor, Department of Chemistry
- SYSTEM HEALTH
The focus of my research is on nervous system injury, repair, regeneration and modulation. My laboratory has pioneered the monitoring and quantitative analysis of neuro-electrophysiological signals in order to assess the electrical integrity of nervous system at various stages. My mostly translational research projects involve acute hypothermia treatment as well as stem cell replacement therapy derived from human embryonic stem cells, human iPS cells, and direct conversion by trans-differentiation of human adult cells. These stem cells are also modified to induce overexpression of neurotrophic factors in order to modulate the lesion microenvironment and stimulate endogenous regenerative responses post injury. A part of my laboratory is also investigating application of upconversion nanoparticles in conjunction with near infrared illumination system for the neuromodulation applications both in vitro and in vivo. The goal is to develop a semi-invasive tool for stimulating or inhibiting, with spatio-temporal precision, neuropathways situated deep in the nervous system (spinal cord, motor cortex, vagus nerve, etc.) selectively.
In addition, my laboratory is studying the adult central nervous system capabilities of adaptive changes enabling reorganization and plasticity in spared neuropathways following neuro-trauma. I have also used different imaging techniques to monitor anatomical changes in contused spinal cord architectures and brain at various time points enabling me to identify spared fibers (anatomically intact but nonfunctional neuropathways) and tracking the extent of secondary injury to determine the therapeutic benefits of the various treatment strategies.
Research Interests
Upconversion Nanoparticles, Optogenetics, Neuromodulation, Neuroplasticity, Neurotrauma, Neuro-electrophysiology, Stem Cell Replacement Therapy, Hypothermia.
Highlights
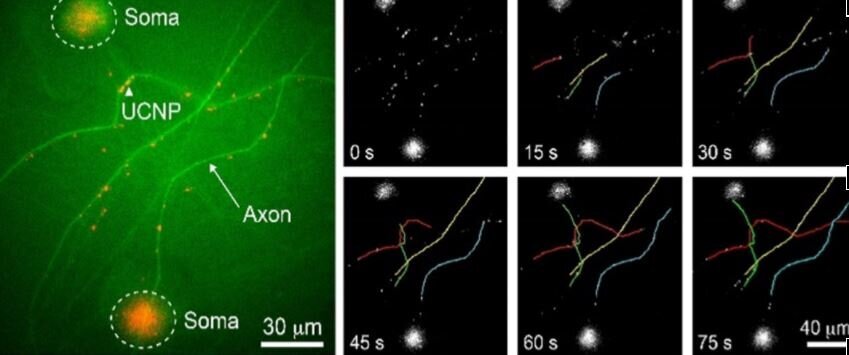
Fig 1: This Figure below shows real-time tracking of intracellular dynein movement with UCNP labels. Left: Luminescence microscopy: the green fluorescence signals represented calcein labelled soma and axons. The red dots depicted UCNP emission in the visible spectrum. Middle: Time lapse (0/15/30/45/60/75 s) tracking of four individual upconversion luminescence spots over long distance (100μm). The trajectories represented by the four emission spots (yellow, green, blue and red) did match with the axon shape on the left, indicating that UCNPs were traveling along the axon track. Right: Histogram of dynein average speed in presence (blue) or absence (brown) of dynein inhibitor, ciliobrevin D.
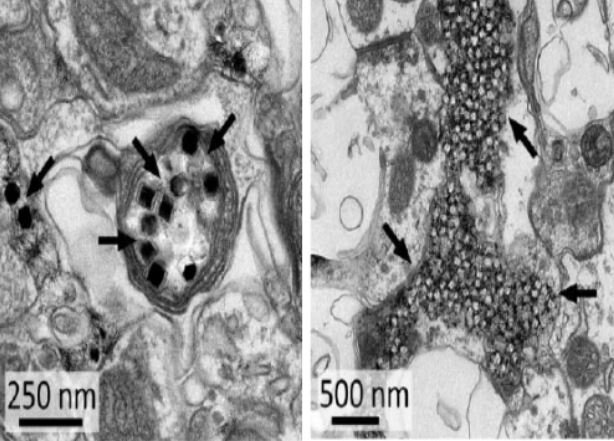
Fig 2: This figure shows electron micrographs of Upconversion Nanoparticles (UPCNs) distribution at the injection site in the Central Nervous System. Black arrows show clusters of UCNPs. Right figure shows most of UCNPs in extracellular space and Left figure shows the uptake of UCNPs by axons.
.jpg)

